Background: Hypomethylating agents (HMAs) are the standard of care in patients with higher-risk myelodysplastic syndrome (MDS), but the survival of patients after failure of HMA therapy is poor at approximately 4 to 6 months. Expression of PD-1 and PD-L1 was increased in CD34 positive cells from patients with MDS with further upregulation following HMA therapy and HMA failure (Yang H, Leukemia 2014). Pembrolizumab is a humanized monoclonal antibody targeting PD-1, thus blocking its interaction with ligands PD-L1 and PD-L2. A phase 1b, multicohort study of pembrolizumab in advanced hematologic malignancies showed a small number of patients with long-term survival and no immune-mediated adverse events in the higher-risk MDS cohort (Garcia-Manero G, Blood 2016). We report final results from a phase II clinical trial evaluating the safety and clinical activity of azacitidine and pembrolizumab in patients with higher-risk MDS after failure of hypomethylating agent therapy.
Methods: Adult patients with intermediate-1- or higher-risk disease by the International Prognostic Scoring System (IPSS) with adequate renal and hepatic function and no prior stem cell transplantation, active autoimmune disease, or immunodeficiencies were eligible for the study. For this HMA failure cohort, patients had to not respond to, progress on, or relapse after at least 6 cycles of HMA therapy. Patients received azacitidine 75 mg/m2 intravenously (IV) or subcutaneously daily for 7 days every 28-day cycle and pembrolizumab 200 mg IV starting on cycle 1 day 1 and every 21 days thereafter independent of the azacitidine dosing schedule. The endpoints were overall response rate, survival, and safety. The criteria for early trial termination included an overall response rate (ORR) < 20%, incidence of grade 3-4 adverse events (AEs) > 30%, poor adherence to protocol and regulatory requirements, severe and adverse drug reactions, and plans to modify or discontinue development of the study drug. Clinical trial information: NCT03094637.
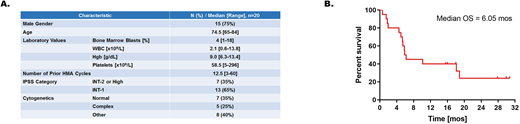
Results: At data cut-off (July 2020), 20 patients with HMA failure have been enrolled and treated with combination azacitidine and pembrolizumab with 2 patients continuing on treatment in cycles 30 and 33. The median age of patients treated is 74.5 years, and additional patient characteristics are shown in Figure A. The overall response rate is 25%, with 1 patient achieving complete remission (CR), 2 patients attaining marrow CR (mCR), and 2 patients showing hematological improvement of platelets (HI-P). One of the responders has normal cytogenetics, 1 has del(20q), 1 has del(5q), and 1 has complex karyotype. The most frequently observed mutations in the 5 responding patients are ASXL1 and SETBP1 in 2 patients each. Interestingly, the patient on treatment in cycle 33 has 2 separate TP53 mutations and continues to have stable disease with sustained transfusion independence. With a median follow-up time of 27.9 months, the overall survival is 6.1 months (Figure B).
Treatment is overall well-tolerated. The most common treatment-related adverse events (all grades) observed are neutropenia (40%), pneumonia (35%), constipation (30%), and febrile neutropenia (25%). Two patients died in the first 60 days while receiving treatment from the unrelated causes of septic shock and fungal pneumonia.
Conclusions: This phase II trial suggests that the combination of azacitidine and pembrolizumab was relatively safe and well-tolerated in patients who had previously failed HMA therapy. Though no significant improvement in overall survival was observed, combination therapy may have antitumor activity in certain HMA failure patients, resulting in sustained responses in some high-risk individuals.
Borthakur:PTC Therapeutics: Consultancy; GSK: Research Funding; Jannsen: Research Funding; Abbvie: Research Funding; BioLine Rx: Consultancy; BioTherix: Consultancy; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy; Incyte: Research Funding; AstraZeneca: Research Funding; Argenx: Consultancy; FTC Therapeutics: Consultancy; Curio Science LLC: Consultancy; Oncoceutics: Research Funding; Xbiotech USA: Research Funding; Polaris: Research Funding; BioLine Rx: Research Funding; Cyclacel: Research Funding; BMS: Research Funding; Novartis: Research Funding; PTC Therapeutics: Research Funding. Daver:Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cortes:Immunogen: Research Funding; Merus: Research Funding; Arog: Research Funding; Amphivena Therapeutics: Research Funding; Astellas: Research Funding; Telios: Research Funding; BioPath Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sun Pharma: Research Funding; Takeda: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; BiolineRx: Consultancy, Research Funding. DiNardo:MedImmune: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Syros: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria; Calithera: Research Funding; Novartis: Consultancy; Jazz: Honoraria; Takeda: Honoraria. Jabbour:Amgen: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding. Alvarado:FibroGen: Research Funding; BerGenBio ASA: Research Funding; MEI Pharma: Research Funding; Daiichi-Sankyo: Research Funding; Tolero Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; Jazz Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding. Andreeff:Daiichi-Sankyo; Jazz Pharmaceuticals; Celgene; Amgen; AstraZeneca; 6 Dimensions Capital: Consultancy; Centre for Drug Research & Development; Cancer UK; NCI-CTEP; German Research Council; Leukemia Lymphoma Foundation (LLS); NCI-RDCRN (Rare Disease Clin Network); CLL Founcdation; BioLineRx; SentiBio; Aptose Biosciences, Inc: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Daiichi-Sankyo; Breast Cancer Research Foundation; CPRIT; NIH/NCI; Amgen; AstraZeneca: Research Funding. Bose:Kartos Therapeutics: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding; NS Pharma: Research Funding; Astellas Pharmaceuticals: Research Funding; Constellation Pharmaceuticals: Research Funding; Celgene Corporation: Honoraria, Research Funding; Promedior, Inc.: Research Funding; Incyte Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer, Inc.: Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding. Jain:Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Incyte: Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Cellectis: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; ADC Therapeutics: Research Funding; TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kantarjian:Pfizer: Honoraria, Research Funding; Sanofi: Research Funding; Abbvie: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Adaptive biotechnologies: Honoraria; Aptitute Health: Honoraria; BioAscend: Honoraria; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Jazz: Research Funding; Immunogen: Research Funding; Delta Fly: Honoraria; Janssen: Honoraria; Oxford Biomedical: Honoraria; Ascentage: Research Funding; BMS: Research Funding; Amgen: Honoraria, Research Funding. Garcia-Manero:Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy; Novartis: Research Funding; H3 Biomedicine: Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amphivena Therapeutics: Research Funding; Merck: Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Onconova: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.